Science
Kiran Musunuru Outlines Groundbreaking Gene Therapy Advances at ESGCT
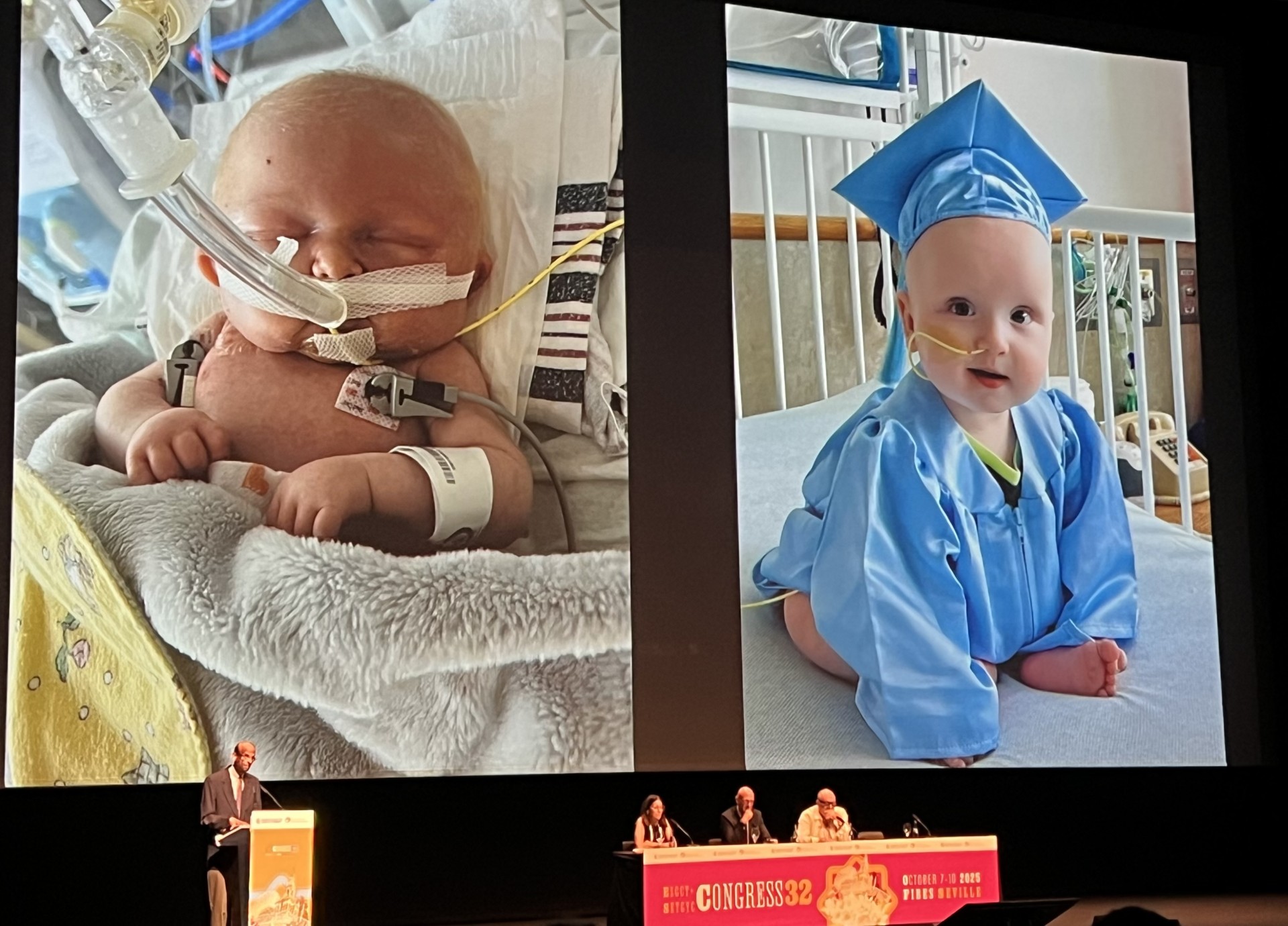
SEVILLE – The annual conference of the European Society of Gene & Cell Therapy (ESGCT) kicked off this week in the capital of Andalusia, showcasing significant advancements in the field of gene therapy. A highlight of the opening plenary session was an update from Kiran Musunuru, MD, PhD, from the Perelman School of Medicine at the University of Pennsylvania, regarding the remarkable case of infant KJ Muldoon, known as “Baby KJ,” who received groundbreaking treatment for a rare genetic disorder.
Musunuru presented a roadmap for future developments in N-of-1 gene editing therapies, drawing upon insights gained from KJ’s diagnosis and treatment over the past year. He emphasized the urgent need for innovative therapies, particularly for patients with rare diseases, stating, “We’d have to personalize the therapies to a degree that had never been done before.”
In 2014, Musunuru’s lab pioneered the therapeutic suppression of the PCSK9 gene using CRISPR-Cas9 technology, a breakthrough that has since been commercially developed by Verve Therapeutics, a company he co-founded. The potential of this technology continues to evolve, reflected in the increasing support for tailored treatments for patients with unique genetic mutations.
Addressing Critical Health Challenges
KJ was born with a severe deficiency of the liver enzyme CPS1, resulting in ammonia levels that were approximately 30 times higher than normal. This condition significantly increases the risk of brain injury and infant mortality. Musunuru described KJ’s situation as “the most severe form of the most severe disorder” within the urea cycle category. Initially, KJ was admitted to the Children’s Hospital of Philadelphia (CHOP) not for treatment but to await a liver transplant.
In collaboration with Rebecca Ahrens-Niklas, MD, PhD, Musunuru’s team worked swiftly to develop a bespoke treatment plan. They identified KJ’s specific CPS1 mutations and employed lentivirus-transduced cell lines to evaluate various gene editing constructs. “It was all about speed; we couldn’t let perfect be the enemy of the good,” Musunuru remarked.
Within weeks, the team pinpointed an effective guide RNA and collaborated with experts, including Ben Kleinstiver, PhD, from Mass General Hospital, to enhance the editing efficiency of their approach. Their public-private partnership included resources from organizations such as Aldevron, IDT, and The Jackson Laboratory, demonstrating a collective commitment to addressing this urgent health crisis.
Regulatory Milestones and Positive Outcomes
Four months after KJ’s birth, Musunuru’s team engaged in a pre-IND (investigational new drug) meeting with the U.S. Food and Drug Administration (FDA), which expressed strong support for the initiative. Toxicology and dosing studies were completed promptly, and clinical production of necessary reagents was finalized shortly after the six-month mark.
In a significant achievement, KJ received his first dose of a custom lipid nanoparticle-based gene editor at the age of seven months. The treatment protocol included two additional doses, administered 22 and 62 days later. Remarkably, KJ experienced only mild side effects, and subsequent tests indicated noticeable improvements in his health, as he grew from the 9th percentile to the 40th percentile in growth metrics.
Musunuru underscored the importance of caution in communicating treatment outcomes, stating, “I avoid using the word cure. We have a responsibility not to exaggerate and give undue hope to the families.” Although KJ’s condition is now more manageable, he continues to require careful monitoring.
The case of Baby KJ serves as a powerful illustration of the potential for personalized gene therapy to transform clinical care. Musunuru and his colleagues are now devising a master protocol for a clinical trial that would include patients with various urea cycle disorders. This initiative aims to transition from N-of-1 studies to more extensive platform trials, potentially paving the way for broader applications of gene editing therapies.
As Musunuru concluded his presentation, he expressed gratitude to KJ’s parents and shared a touching “before and after” photograph of the young patient, capturing the remarkable journey from his initial admission to CHOP to his current improved state. The ongoing developments in gene therapy, as presented at ESGCT 2025, signal a promising future for patients with rare genetic disorders.
-

 Lifestyle3 months ago
Lifestyle3 months agoLibraries Challenge Rising E-Book Costs Amid Growing Demand
-

 Sports3 months ago
Sports3 months agoTyreek Hill Responds to Tua Tagovailoa’s Comments on Team Dynamics
-

 Sports3 months ago
Sports3 months agoLiverpool Secures Agreement to Sign Young Striker Will Wright
-

 Lifestyle3 months ago
Lifestyle3 months agoSave Your Split Tomatoes: Expert Tips for Gardeners
-

 Lifestyle3 months ago
Lifestyle3 months agoPrincess Beatrice’s Daughter Athena Joins Siblings at London Parade
-

 World2 months ago
World2 months agoWinter Storms Lash New South Wales with Snow, Flood Risks
-

 Science3 months ago
Science3 months agoTrump Administration Moves to Repeal Key Climate Regulation
-

 Business3 months ago
Business3 months agoSoFi Technologies Shares Slip 2% Following Insider Stock Sale
-

 Science3 months ago
Science3 months agoNew Tool Reveals Link Between Horse Coat Condition and Parasites
-

 Science2 months ago
Science2 months agoSan Francisco Hosts Unique Contest to Identify “Performative Males”
-

 Sports3 months ago
Sports3 months agoElon Musk Sculpture Travels From Utah to Yosemite National Park
-

 Science3 months ago
Science3 months agoNew Study Confirms Humans Transported Stonehenge Bluestones








